Aim¶
To show the random zigzag motion of small particles in a liquid.
Subjects¶
4D10 (Brownian Motion)
Diagram¶
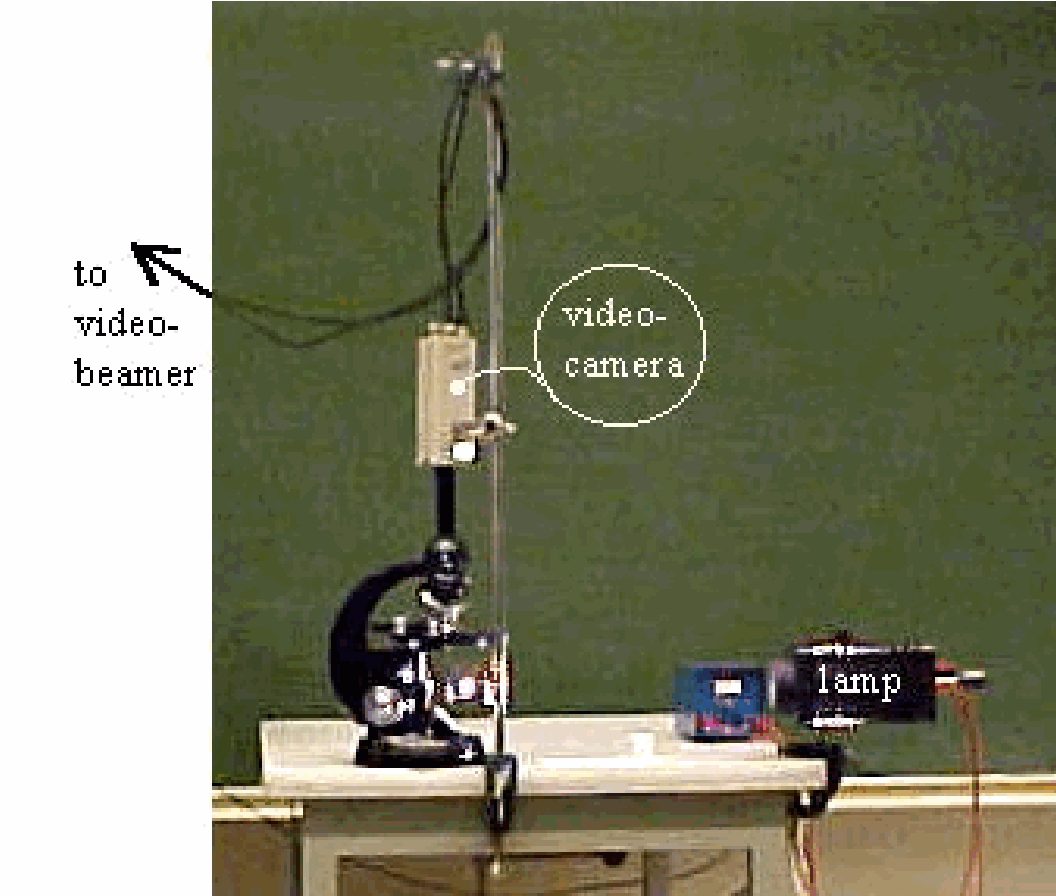
Equipment¶
Microscope.
Microscope slide on which a shallow “well” is made by means of thin adhesive tape.
Thin cover glass (we use ).
Solution op polystyrene latex particles, normally used as calibration particles in microscopic analysis (we use a solution with mean particle size ).
Video projector.
Presentation¶
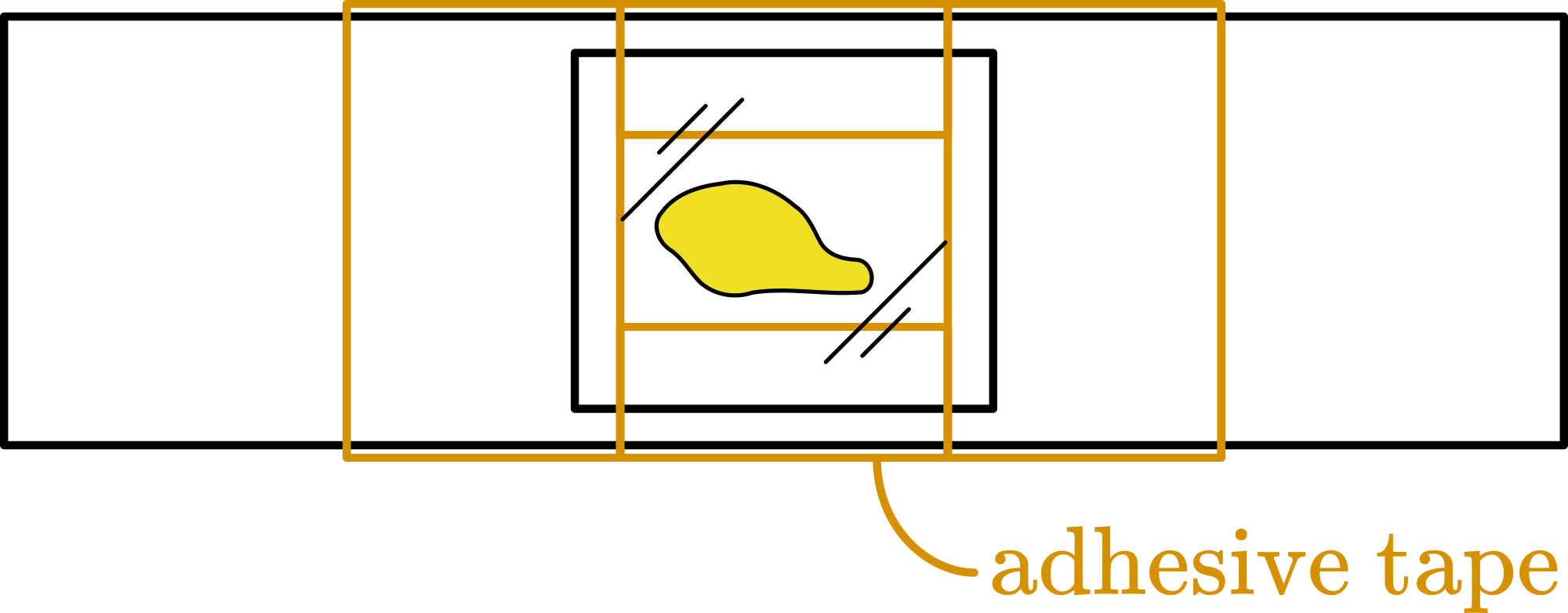
To show Brownian motion we use a solution of polystyrene latex particles in distilled water (see Equipment). A small drop of this solution is placed in the “well” on the microscope slide. The “well” is covered by the thin cover glass (see Figure 2).
This combination is placed on the stage in the clutch mechanism of the microscope. The eyepiece of the microscope is removed and the video-camera (without lens) is placed on the tube (see Diagram). Using a 40x objective will make individual particles clearly visible when the sub-stage illumination is switched on. The zigzag movement of the particles can be observed now.
Explanation¶
This demonstration of these irregular motions by Robert Brown (1828) provides evidence for the basic hypotheses of the kinetic theory of gasses. These hypotheses postulate the microscopic particle nature of gasses to explain its macroscopic properties.
The random zigzag movement can be explained as being the result of the bombardment of the many molecules of the liquid (which are too small to be visible themselves). To “prove” this, a simulation can be shown. ISSUE: simulation needed.
Remarks¶
The drop in the “well” should not touch the walls of the well, because otherwise leakage of liquid is inevitable and this will show as a strong flow in your liquid. So really get a drop something like in Figure 2.
We get the best image when we focus not on the surface of the liquid but at a certain depth.
Sources¶
Mansfield, M and O’Sullivan, C., Understanding physics, pag. 247.