Aim¶
To show an apparent violation of the second law of thermodynamics
Subjects¶
4F10 (Entropy)
Diagram¶

Equipment¶
Petri dish.
Transparent oil (e.g. sunflower oil).
Transparent cylinder, containing a second, white cylinder inside that can turn around the common axis.
Glycerine.
Syringe with long needle.
Red ink.
Presentation¶
As an introduction a Petri dish is placed on the overhead projector. The dish contains a layer of (transparent) oil. By means of the syringe a large drop of dark ink is spouted into the oil (see Figure 2 a). Using a flat spatula one rotation is made in the fluid. The drop of dark ink breaks into smaller drops (Figure 2b).
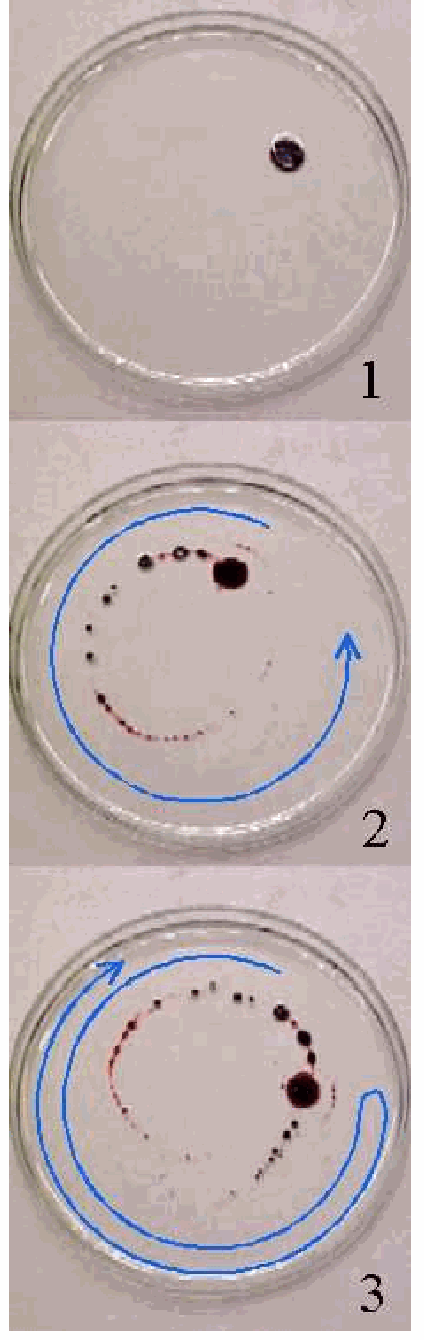
Now it is suggested to the students to turn the spatula in the liquid into the opposite direction, in order to repair the original drop of ink. This action is performed, but the result is that still more destruction is done to the inkdrops (Figure 2c).
Next the experiment with the plastic cylinder (see Diagram) is done:
The space between the two concentric cylinders is filled with glycerine and a column of ink is introduced into the glycerine using the syringe with a long needle.Then the inner cylinder is rotated one turn and it appears that the ink is mixed with the glycerine. But when a reverse turn is made, the column of ink reforms!
The experiment can be repeated, even making more turns: the column reforms when the same number of reverse turns is made.
Explanation¶
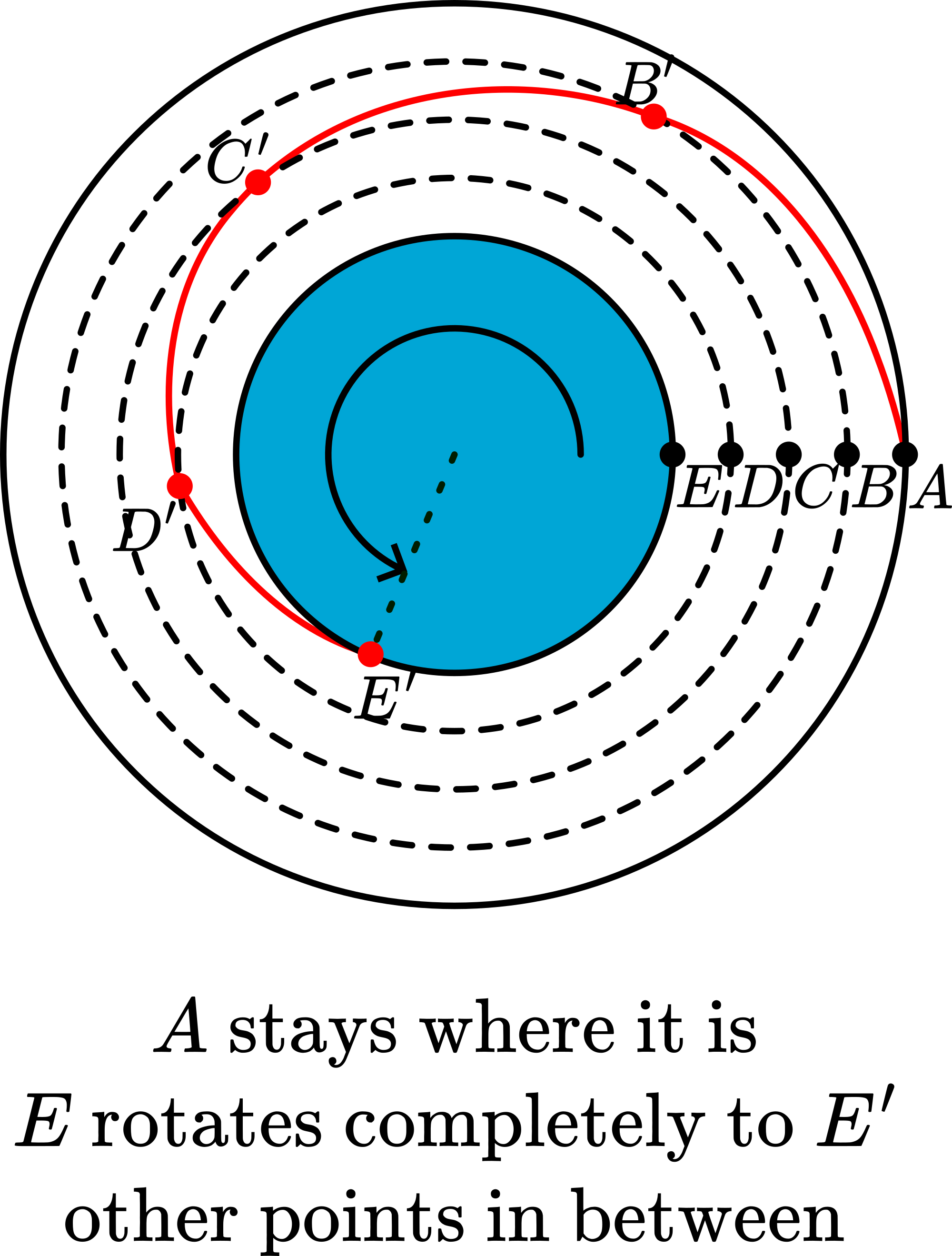
There is no violation of the entropy law at work in this demonstration. The spreading out of the ink does not represent any increase in entropy or disorder. An enlarged top view of the space between the two cylinders (see Figure 3) shows that molecules on the inner edge of the liquid rotate through angles different from those in the middle or outer edge. There is only a laminar displacement; no mixing occurs. And so the original vertical line is restored when the rotation is reversed.
Remarks¶
When the glycerine is too fluid it is advised to refrigerate the system before using it.
The inner cylinder is constructed heavy enough to prevent it will float in the glycerine.
Sources¶
Ehrlich, Robert, Turning the World Inside Out and 174 Other Simple Physics Demonstrations, pag. 124-125
Freier, George D. and Anderson, Frances J., A demonstration handbook for physics, pag. H31
Mansfield, M and O’Sullivan, C., Understanding physics, pag. 290