Aim¶
To introduce how matter waves can be associated to Bohr’s model of an atom; a classical analogy.
Subjects¶
3B22 (Standing Waves)
7A50 (Wave Mechanics)
7B50 (Atomic Models)
Diagram¶
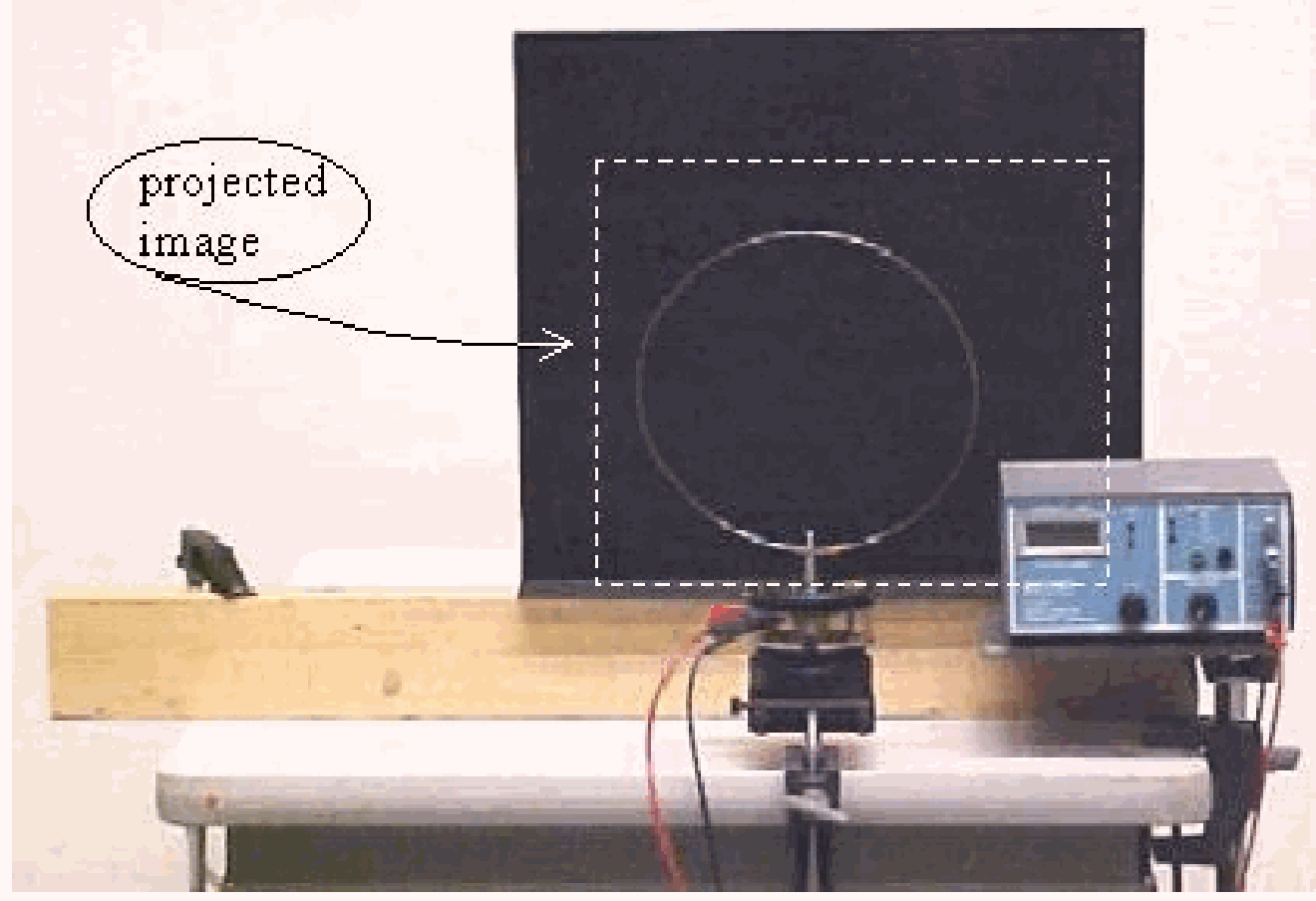
Equipment¶
Wire loop of spring steel (round, 1mm), bend into a circular loop with a diameter of around 25 cm, and fixed to a banana plug (Pasco SF-9405).
Mechanical wave driver (Pasco SF-9324), actually an adapted loudspeaker.
Signal generator.
Black screen.
Camera and beamer to show the demonstration to a large audience.
Safety¶
The loop is fixed to the wave driver. When the loop is agitated heavily it is possible that it loosens itself. Then a free end sweeps around dangerously. So fix the loop tightly.
Presentation¶
The wire loop is fitted to the mechanical wave driver shaft. The wave driver is connected to the signal generator. The image of wire loop and display of the frequency of the driving generator is projected (see Diagram).
Start at low frequency (around ) and low amplitude, making the loop starting to vibrate. Increase the frequency to see various modes of standing waves in the circular loop. (At higher frequencies the amplitude of the signal generator has to increase to obtain visible amplitude in the oscillating wire loop.) We observe:
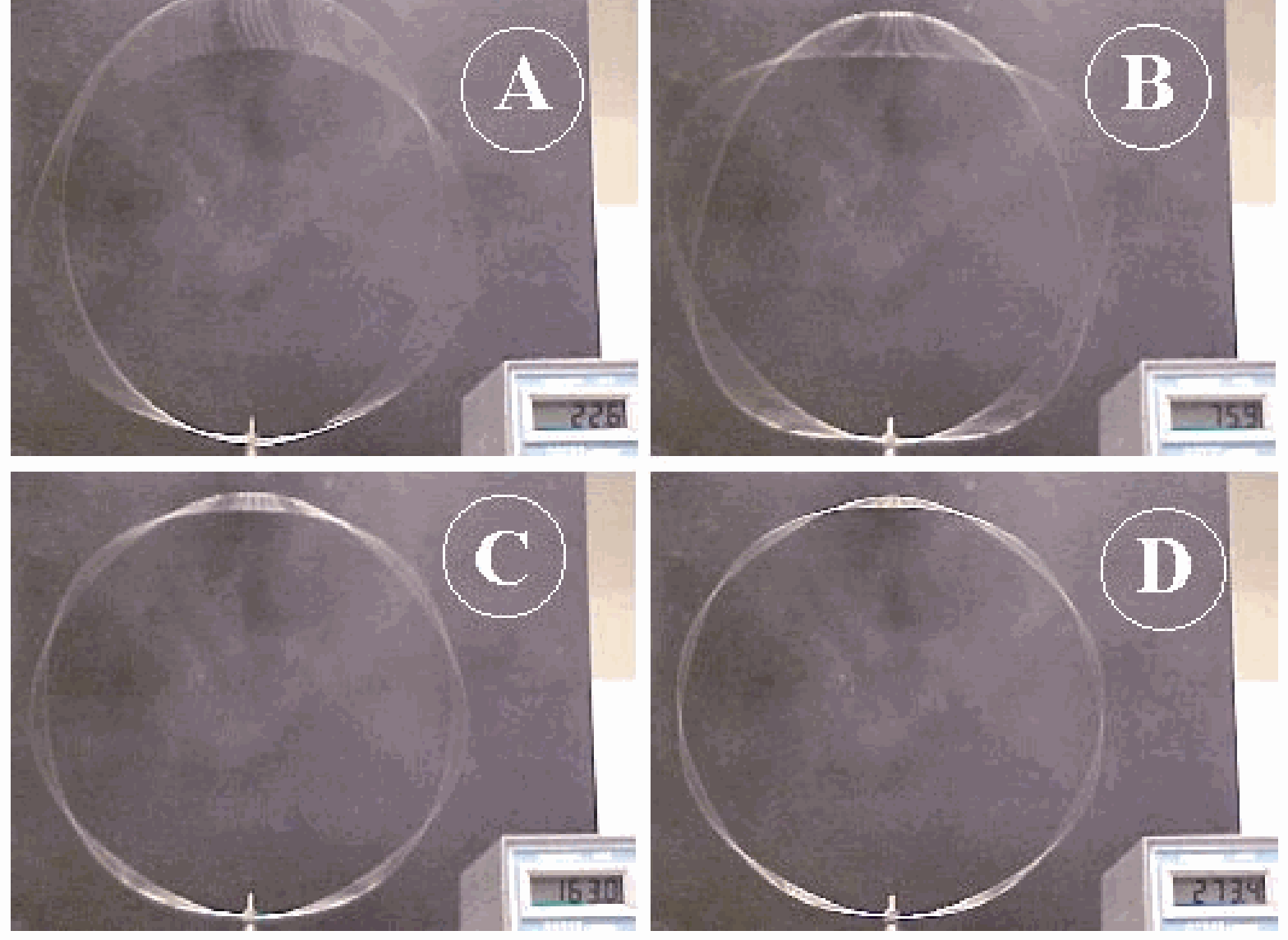
2 nodes and anti-nodes at ;
3 nodes and anti-nodes at (very large amplitude) (see Figure 2A);
4 nodes and anti-nodes at ;
5 nodes and anti-nodes at (see Figure 2B);
7 nodes and anti-nodes at (see Figure 2C);
9 nodes and anti-nodes at (see Figure 2D);
-11 nodes and anti-nodes at (this last one is not so good visible to a larger audience due to its low amplitude).
Explanation¶
According to Bohr electrons move in circles.
DeBroglie argued that the “electron wave” was a circular standing wave that closes in itself (in order to obtain constructive interference). So, for persisting waves: . This is what we observe in our demonstration.
DeBroglie: and we get: . In this way the ad hoc quantized orbits of Bohr are derived form deBroglie. This “shown” wave-particle duality is at the root of atomic structure.
(In discussing the analogy it should be remembered that the “electron wave” is not in reality a standing wave along a line, but it extends through all space.)
Remarks¶
For the lower frequencies, and , the successive patterns ( 2,3 and 4 half wavelengths) are “logic” when we suppose that is obtained at around . For higher number of nodes/anti-nodes this logic order is apparently a different one.
Sources¶
Giancoli, D.G., Physics for scientists and engineers with modern physics, pag. 971-972
Mansfield, M and O’Sullivan, C., Understanding physics, pag. 573-574
Meiners,H., Physics demonstration experiments, part 2, pag. 1185-1188