02 Boiling Water at Reduced Pressure#
Aim#
To show that at pressures below \(105 \mathrm{~Pa}\) water boils below \(100^\circ \mathrm{C}\).
Subjects#
4C33 (Vapor Pressure)
Diagram#
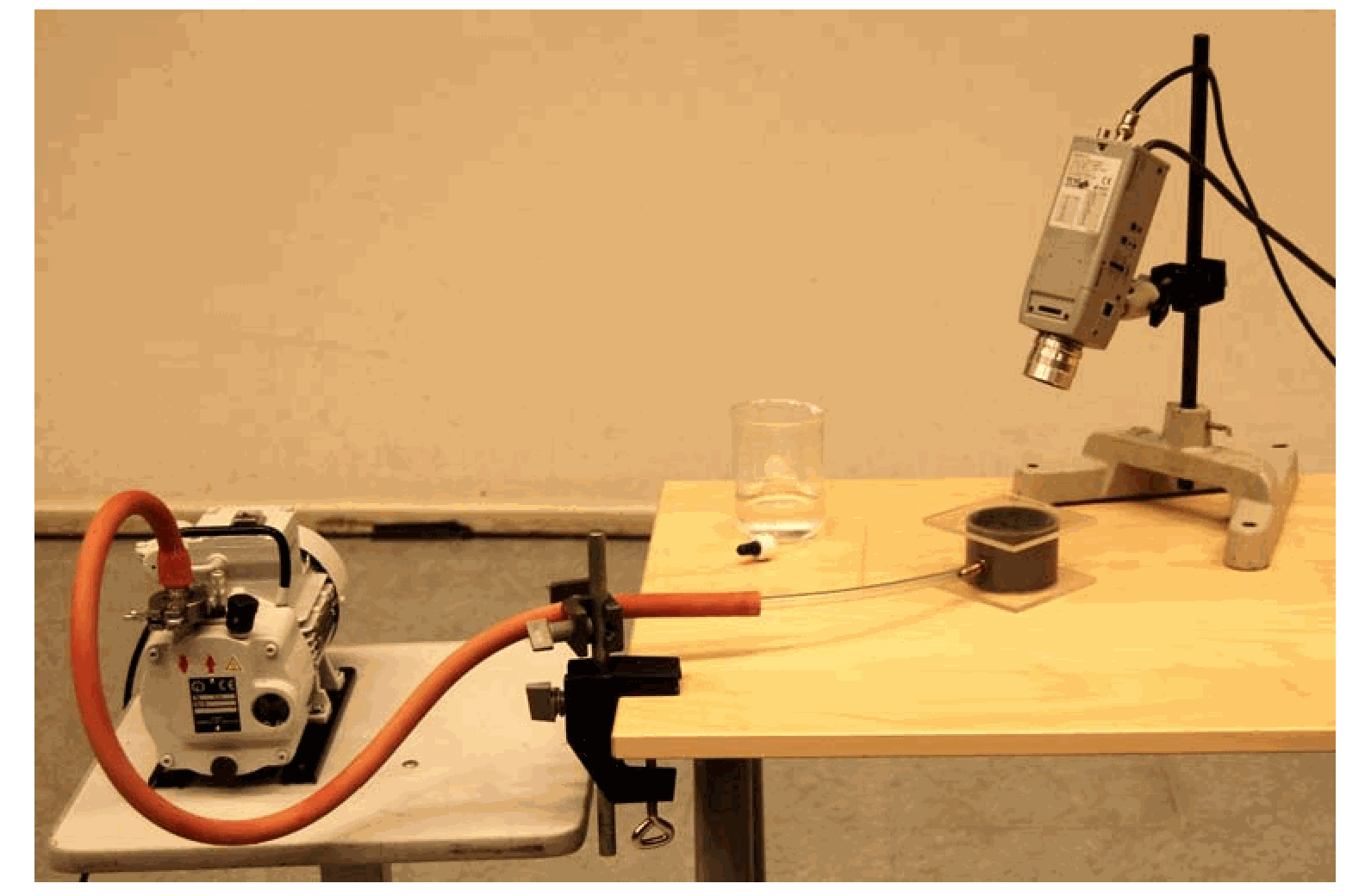
Fig. 413 .#
Equipment#
Large metal tray \(\left(1 \times 1 m^{2}\right)\).
2 I flask, with round bottom, half filled with water.
Rubber stopper, with cock and temperature sensor with digital read-out.
Support clamp.
Gas flame.
Protective gloves.
Ice-water.
Video-camera and projector to project an image of the flask.
Safety#
Perspex safety screens are placed between the audience and the flask, because implosion can occur. (See the two screens, that are placed on the table, in the Diagram above.)
Don’t use a flat bottomed flask. Such a flask is more likely to implode under the reduced pressure.
Use protective gloves when turning the flask upside down.
Always fix the clamp, that holds the flask, firmly!
Presentation#
The 2 I flask is half filled with water. Open the cock. The water is made boiling by means of a gas flame.

Fig. 414 .#
Make it boil rigorously for about one minute to drive out air. Remove the flame and when you see that vapour no longer blows out of the cock, quickly close it. Then invert the flask (see photo in Diagram).
In the beginning the water trapped inside the flask keeps boiling.
After some time the water will stop boiling.
When cold water is poured over the outside of the flask, the water inside the flask starts boiling again. This can be repeated quite a number of times. When there is no longer success with pouring tap-water, ice-water can be tried.
The thermometer shows that the water inside the flask is boiling far below \(100^{\circ} \mathrm{C}\). When using ice-water you can even make the water in the flask boil when it has cooled down to \(20^{\circ} \mathrm{C}\) !
Explanation#
The moment the flask is closed by the rubber stopper, only water and water vapour are present inside the flask. The water-vapour will cool down faster than the water, due to the lower mass of the water-vapour. The resulting lower vapour pressure will make that the water continues to boil in order to saturate the vapour at the saturation pressure that corresponds to the water temperature.
When the temperature becomes lower the process of cooling becomes slower and water temperature and vapour temperature are closer to each other. There is no boiling any longer.
When water is poured over the flask, the vapour temperature quickly lowers. The water temperature inside the flask barely lowers. So, the vapour pressure is lower than the saturation pressure and the water starts boiling again.
Supposing that pouring water can reduce the vapour temperature to (for example) \(30^{\circ} \mathrm{C}\), the process can be repeated until the water inside the flask is cooled to that temperature. Pouring ice-water can reduce the vapour temperature even further, thereby prolonging the described situation.
Remarks#
Stress in your explanation that the change in pressure due to temperature change is so much stronger than in the situation with an ideal gas.
In preparing the flask, fill it with heated water in order to reach \(100^{\circ} \mathrm{C}\) quicker.
Sources#
Sutton, Richard Manliffe, Demonstration experiments in Physics, pag. 217.
Friedrich, Artur, Handbuch der experimentellen Schulphysik, part 4, Wärmelehre, Thermodynamik, Wetterkunde, pag. 125.
