01 Joule’s Experiment#
Aim#
To show the conversion of mechanical energy into heat (and its proportionality with temperature).
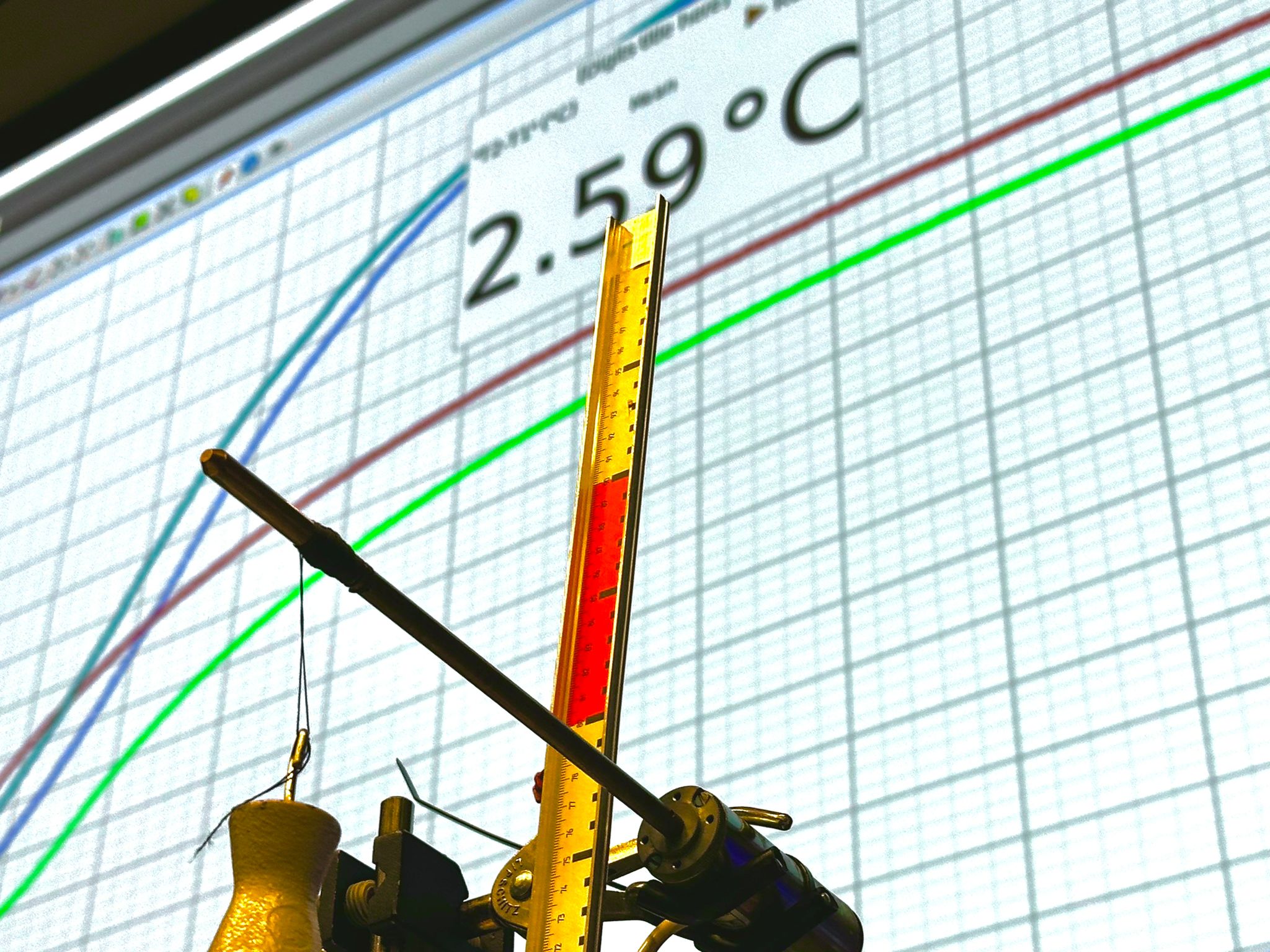
Fig. 370 .#
Subjects#
4B10 (Heat Capacity and Specific Heat)
4B60 (Mechanical Equivalent of Heat)
Diagram#
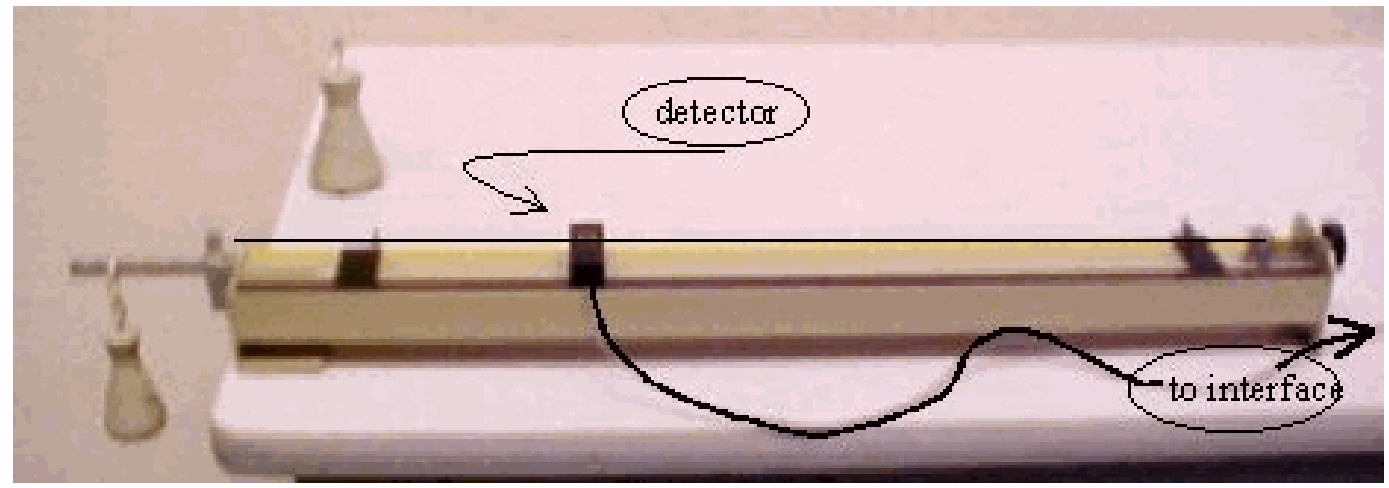
Fig. 371 .#
Equipment#
Electric motor used as generator.
Resistor, with thermocouple attached to it.
mV-meter.
Mass of \(0.5\mathrm{~kg}\), fixed to the axis of the generator by a strong piece of rope.
Piece of foam rubber, to “catch” the falling mass.
Two rulers, \(1\mathrm{~m}\) each.
Two cursors on each ruler.
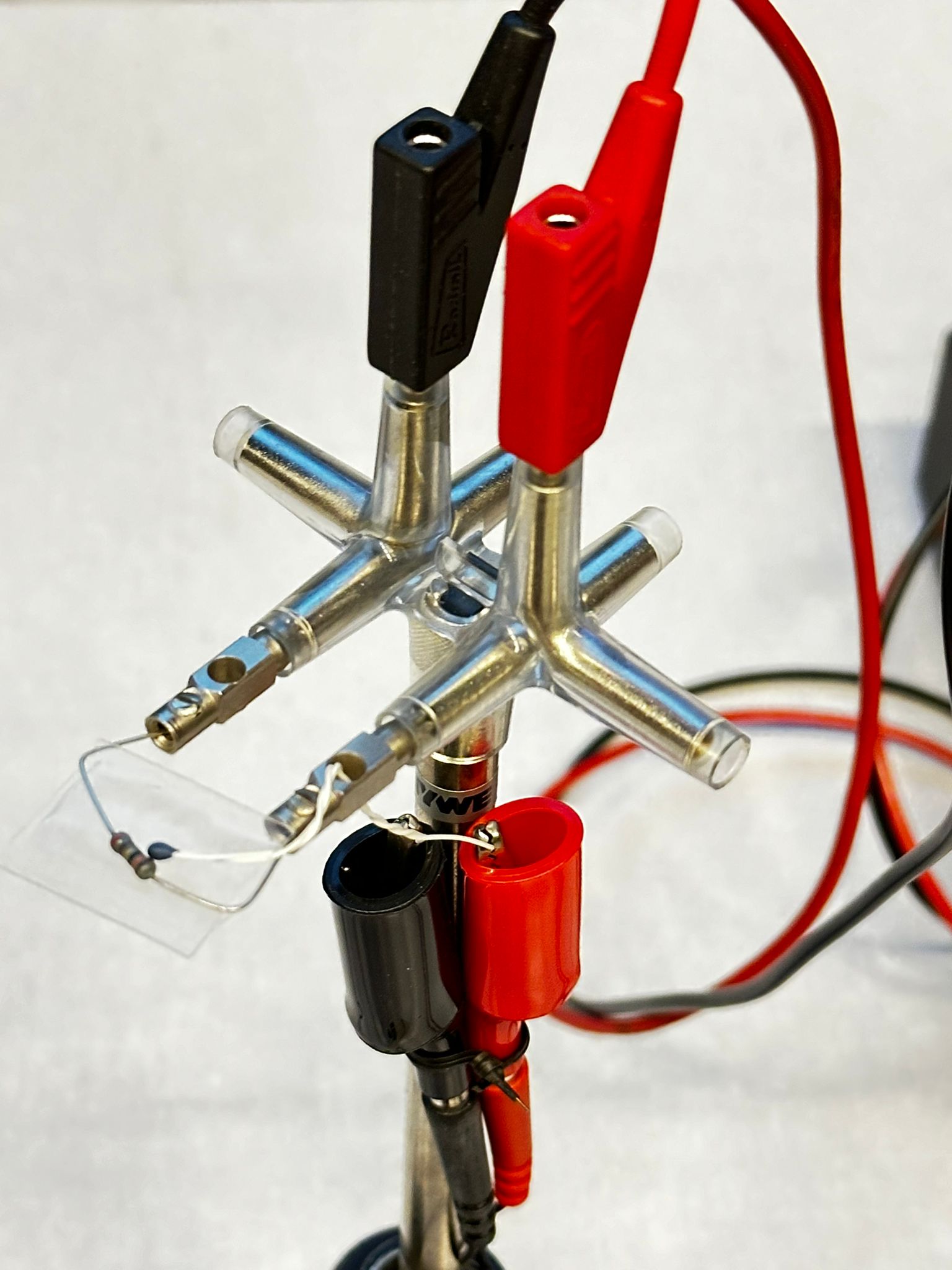
Fig. 372 .#
Presentation#
Set up the equipment as shown in Diagram. Explain the set-up to the students and show that the thermocouple that is fixed on the resistor \(R\), measures temperature, by touching the thermocouple with your fingers: the \(\mathrm{mV}\)-meter shows a deflection. 1. Disconnect the generator from the resistance R. By turning the shaft with your hands the mass is lifted \(.75 \mathrm{~m}\) above the ground. Then let it go. It falls with a high speed on the ground (almost free fall).
By turning the shaft, the mass is lifted again . \(75 \mathrm{~m}\) above the ground (position of cursor). The generator is connected to the resistance R. Let it go again. It falls slower towards the ground now. Almost immediately the \(\mathrm{mV}\)-meter (temperature meter) shows a rise in temperature. Read its highest value before it shows the cooling down of the resistor.
Lift the mass to a height of \(1.5 \mathrm{~m}\) above the ground and let it go. The measured temperature-rise will be double the value we measured in the first . \(75 \mathrm{~m}\)-experiment. We conclude a linear relationship between mechanical work and temperature rise (or heat).
Explanation#
Mechanical work is transformed into heat in the system (See Figure 389 1). Part of that heat is dissipated in the resistor.
Fig. 373 .#
Doubling the mechanical energy shows that the temperature rise is doubling. So this experiment shows that the change in internal energy is directly proportional to the corresponding change in temperature.
Remarks#
The mass that drives the system has to go down relatively slow, otherwise the kinetic energy of this mass is too large compared to the energy that enters the system.
The temperature rise of the system is measured in the resistor (part of the total system). Of course there are losses in all transformations involved. We suppose that the efficiencies are constant for different values of \(h\), then \(\Delta T \propto \Delta U\).
When recording the thermocouple \(\mathrm{mV}\)-reading we find a figure as shown in Figure 390
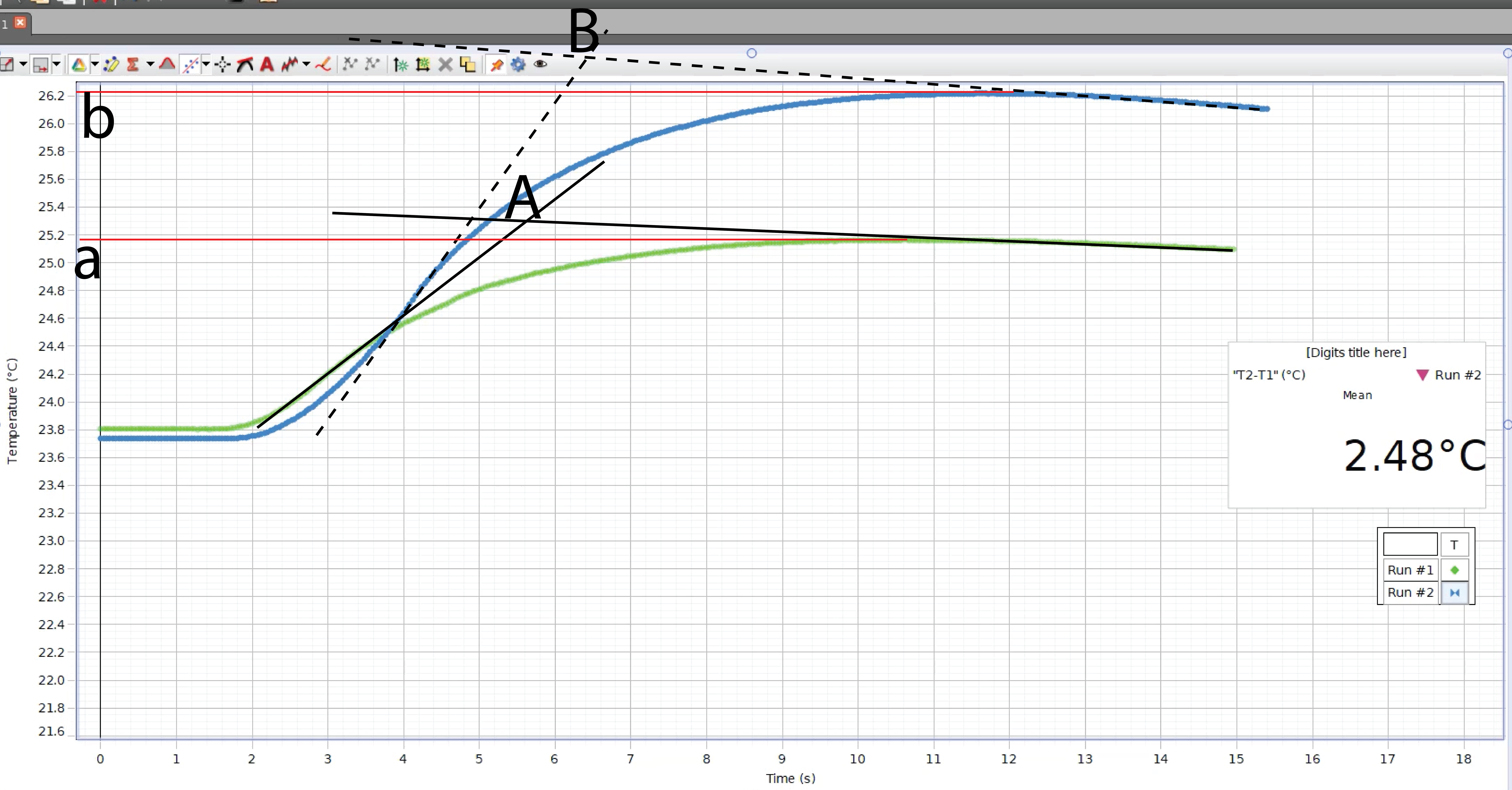
Fig. 374 .#
The two slopes at the beginning of the temperature-rise show the difference in the amounts of heat of the two situations. The end-temperature reached for an ideally isolated resistor can be found by extrapolating the cooling down-lines to these slopes. It can be seen that the ratio between the temperature-readings \(A\) and \(B\) correspond more or less with the ratio of the highest temperatures ( \(a\) and \(b\) ) reached in the real experiment. (Good enough for a demonstration.)
In the set-up a power supply and a two-way switch can be included in such a way that you can drive the generator as a motor to aid in lifting the weight and winding the rope around the axis.
Sources#
Mansfield, M and O’Sullivan, C., Understanding physics, pag. 260-262
Young, H.D. and Freeman, R.A., University Physics, pag. 470-471
